Welcome to the IPEM Generic implant safety procedures (GISPs) Task and Finish Group home page.

UK guidelines for MRI safety recommend that MRI departments refer to the implant manufacturer for advice regarding the MRI safety of scanning patients with an implantable medical device prior to scanning. This process of assuring safety can be time consuming, leading to delays and potential cancellations of a patient’s MRI. The purpose of GISPs is to define a process for managing patients with certain types of implants where the risk from scanning is low. This process incorporates scope for an evidence-based risk-benefit decision to scan some groups of patients under locally-approved conditions, without seeking to identify the exact make and model of the implant and subsequent assurance of MRI safety from the implant manufacturer.
The intention of the task and finish group is to standardise the approach to scanning common implants using MRI. This will be achieved through experts in MR safety collaborating to create a set of nationally agreed standardised GISPs.
Some links to publications can be found below.
Workflow
Generic Implant Safety Procedures are an additional step which can be added to a department’s standard approach to implant safety investigations.
It is expected that this would form the first step in an implant investigation, with an example flow chart shown below:
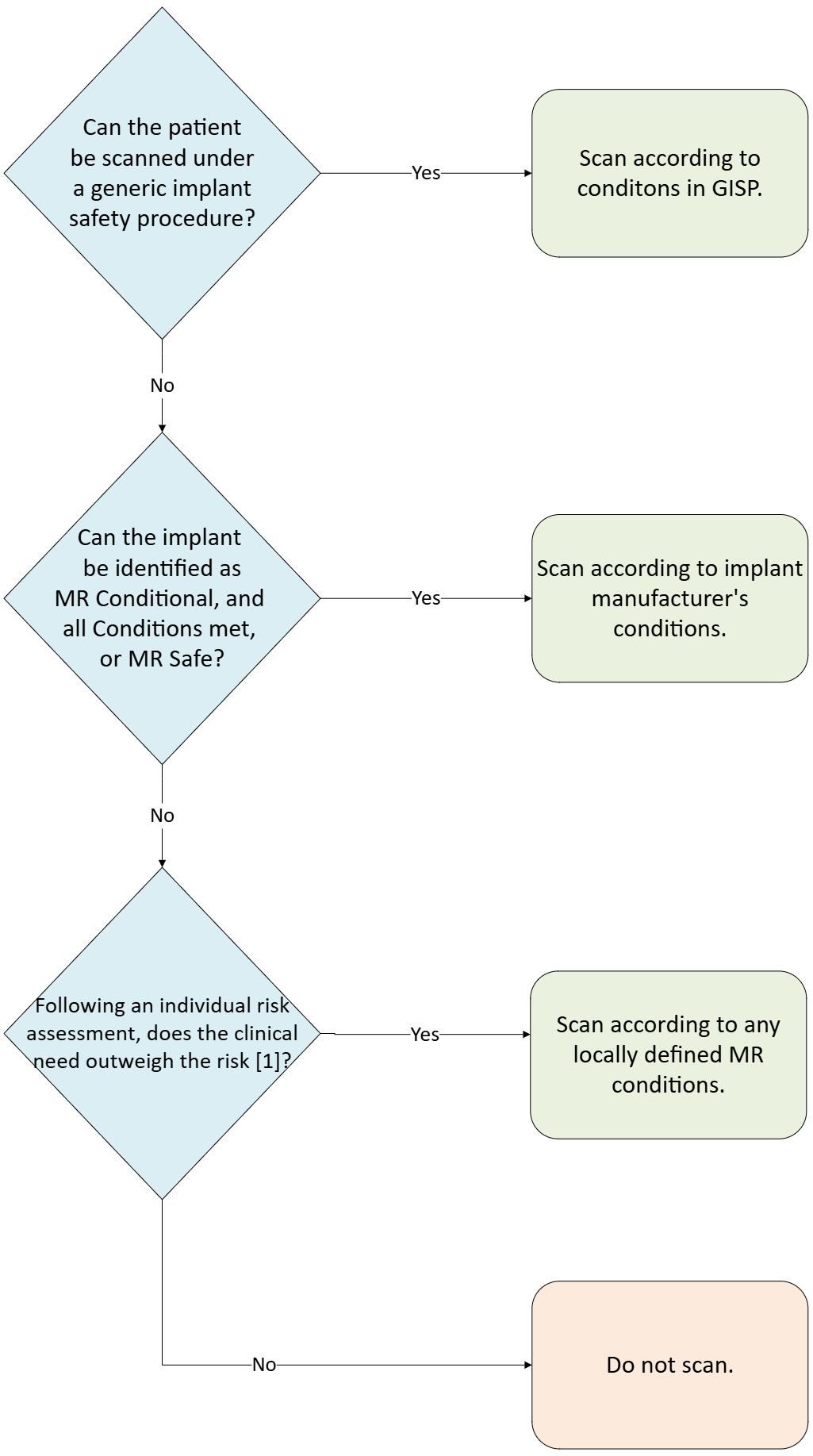
[1] For more information, see MHRA MRI guidelines, Section 4.11.4
Scope
The recommended GISPs on this website have only considered:
- Hydrogen MRI scanning
- Static magnetic fields up to and including 3T
- Horizontal, cylindrical closed-bore scanners.
- Clinically approved (UKCA) MRI scanners
The information contained within the evidence review may be helpful for scanning outside of these conditions, however scanning outside of this scope has not been considered.
Image artefacts have not been considered as part of this process, as the focus has been purely on MRI safety.
Information for patients
If you are a patient reading this and have a concern about having an MRI scan, we strongly recommend you contact the site where your scan is due to take place. Please note local variations to the procedures detailed on this website may apply, therefore please contact the hospital where your appointment is scheduled for clarification.
Disclaimer
The MRI safety information contained within this webpage is intended for use by healthcare professionals working in the United Kingdom. Local sites should review all the information provided, including the evidence review for each GISP, and seek approval for use following their standard governance processes. Local sites should be aware of the risks associated with implementing GISPs, further information can be found in Ashmore et al [2].
GISPs may involve off-label scanning, and sites should be aware of the risks associated with off-label scanning, and have appropriate policies and procedures in place. Further information can be found in Ashmore et. al. [2]
Although the GISPs aim to capture common scenarios, radiographers should still use their professional judgement in cases where they feel that a patient may fall outside of the procedure, and warrants further investigation before scanning. Examples of this could include:
- Devices fitted as part of a trial prior to regulatory approval
- Devices which have clinical issues with their implantation (e.g. snapped, broken etc)
- Excessively large numbers of devices (e.g. 50 embolisation coils in a single patient)
- Additional implants close to the implant covered by a GISP
- Patient who is a poor historian
- Issues where there is not a complete understanding of the GISP such that exclusion/inclusion criteria are not clear
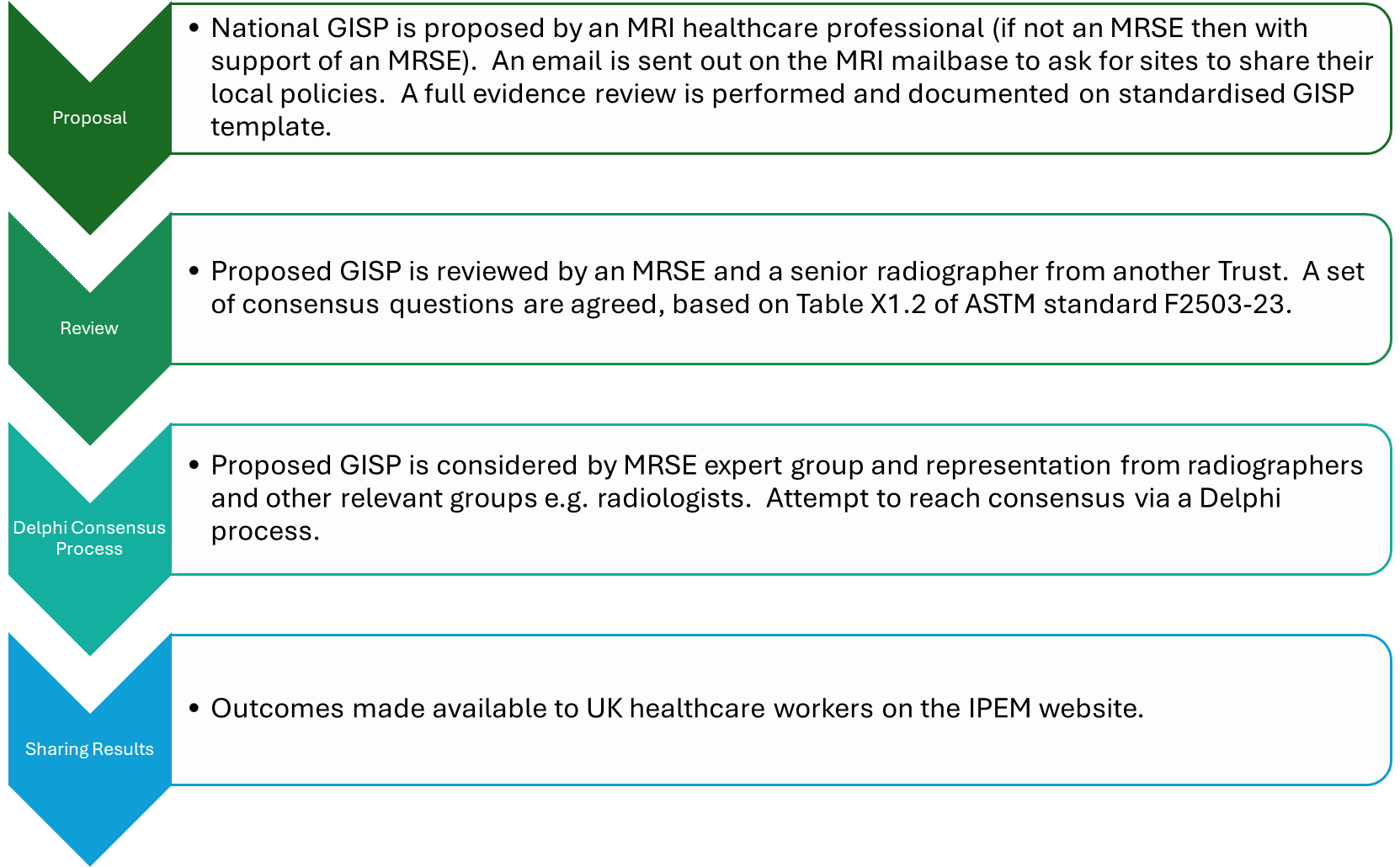
Process for creation of National GISPs
The following steps demonstrate how the national GISPs have been created.
Here is the status of current national GISPs:
|
Intrauterine Devices (IUDs) |
|
|
Heart valves and Annuloplasty Rings |
In Progress |
|
Non-Vascular Stents |
In Progress |
|
Middle Ear Implants |
In Progress |
|
Surgical Clips |
In Progress |
FAQs
I am aware of some evidence or an incident that I think should be considered as it contradicts what is said in a GISP (e.g. a new implant), who do I contact?
Please contact office@IPEM.ac.uk and ask for your feedback to be sent on to the IPEM MR SIG
I am a healthcare professional, and I am interested in becoming more involved with producing GISPs, who should I contact.
Please contact office@IPEM.ac.uk and ask for your details to be sent on to the IPEM MR SIG
Should all UK sites be adopting these national GISPs?
We encourage all UK sites to review the information available on this website to support the implementation of a local GISP. It is recognised that an individual site may have reasons to vary from these national GISPs . Sites should ensure that this has been reviewed by their local governance team before local implementation and are encouraged to adapt these recommendations to suit their local needs, where appropriate.
Why have you not considered MRI scanners outside of the scope given above, e.g. 7T scanning?
The scope above was defined to capture the majority of clinical MRI scanners within the UK. It was also designed around the expertise within the MR safety expert group. The information contained with the detailed evidence review may be beneficial if you are looking to scan an implant outside of the scope of this work.
Is this relevant for research sites?
Although the main focus of this work is for patients undergoing clinical MRI scans, research sites may also find the information useful in informing local practice. Research sites wishing to use these procedures should ensure they have been through all the necessary local approvals before implementation. Further information regarding research use can be found in Ashmore et. al. [2]


